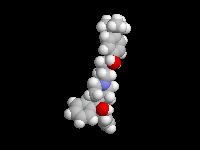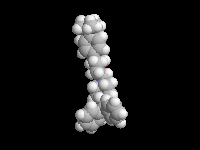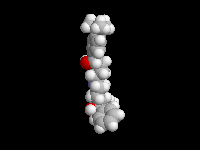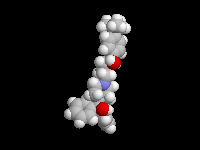
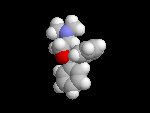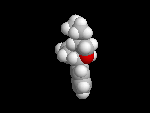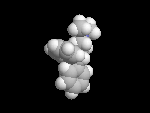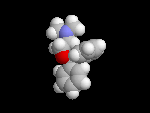

Seldane (terfendine) was designed to have the same pharmacophore as benadryl (circled in purple in the structures at the top of the page), but it was intentially designed to not cross the blood-brain barrier. This largely worked, and seldane was introduced as a next generation antihistamine that did not cause drowsiness. Unfortunately, a few people died of heart failure when they took seldane and seldane was quickly pulled from the market. How could this happen?? In the electrostatic potential surfaces below, note the pharmacophore, namely the two areas of high electron density (red color) that have the same location in three-dimensional space among all the molecules.
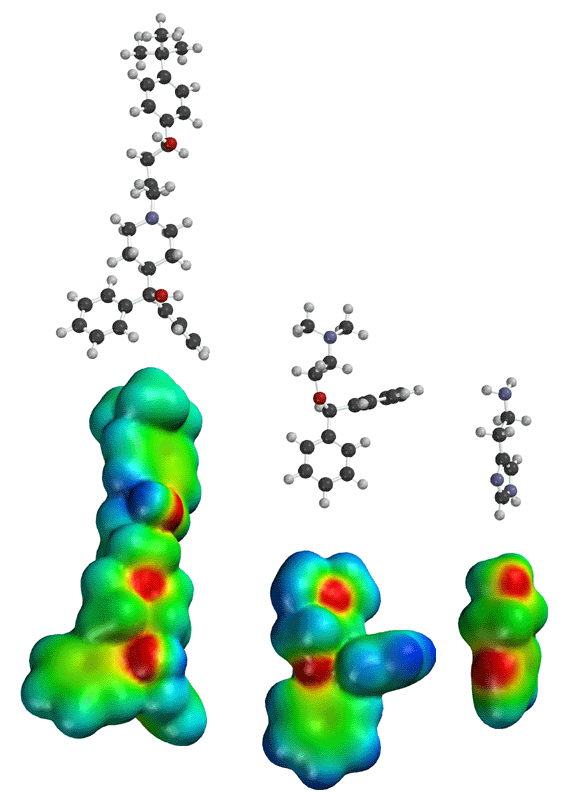
A great deal of scientific detective work determined that the seldane structure (with X = methyl on the uppermost structure) was not actually the antihistamine. In fact, seldane was toxic to the heart!!!!!! In normal people, seldane is circulated through the liver where it is rapidly converted to allegra (fexofenadine), with X = CO2H. The enzyme that carries out this reaction is called P450 and this is the normal mechanism by which molecules are removed from the bloodstream: oxidation. Normally, this conversion happens so fast that there are no ill effects from cardiotoxicity form seldane, and the allegra that is produced acts as an effective antihistamine. However, some patients were taking medications that blocked the action of P450, so these patients could not convert seldane to allegra. They had high levels of seldane in their bloodstream and they died from heart failure. Some common drugs such as anti-fungals and penicillins block P450. Apparently during initial testing, seldane had never be tested on patients taking these medications. Because of seldane and other cases, drug testing now includes a thorough analysis of not only the drug, but all of its breakdown products due to P450. This will never happen again!
When all of this was discovered, allegra was tested then marketed as a totally safe antihistamine that is a big seller today. It just goes to show, with enough research and insight, even difficult problems with side effects can sometimes be solved, leading to better pharmaceuticals.
Review article about Seldane (terfenadine) cardiotoxicity.
A report on how effective allegra (fexofenadine) is at controlling allergies